Electrochemical Characterization of Coordination Complexes
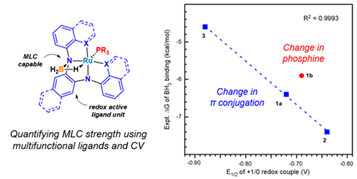
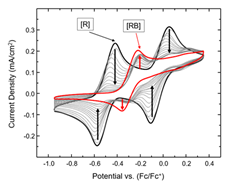
The goal of this collaborative research with the Daly group is to provide fundamental knowledge on how ligand modification impacts substrate binding strength in redox-active metal coordination complexes capable of metal-ligand cooperative binding. A combination of both electrochemical characterization and chemical modeling are used to experimentally determine binding strength for a variety of redox-active ligand systems. This work provides an approach to experimentally quantify subtle changes in binding strength electrochemically, which enables the design and tuning of redox-active coordination complexes for challenging electrocatalytic processes, such as the reduction of carbon dioxide. Future research will utilize metal coordination complexes for this application.
|
|
Spielvogel, K. D.; Stumme, N. C.; Fetrow, T. V.; Wang, L.; Luna, J. A.; Keith, J. M.; Shaw, S. K.; Daly, S. R., Quantifying Variations in Metal–Ligand Cooperative Binding Strength with Cyclic Voltammetry and Redox-Active Ligands. Inorganic Chemistry 2022. DOI: 10.1021/acs.inorgchem.1c03014
Spielvogel, K. D.; Luna, J. A.; Loria, S. M.; Weisburn, L. P.; Stumme, N. C.; Ringenberg, M. R.; Durgaprasad, G.; Keith, J. M.; Shaw, S. K.; Daly, S. R., Influence of Multisite Metal-Ligand Cooperativity on the Redox Activity of Noninnocent N2S2 Ligands. Inorg Chem 2020, 59 (15), 10845-10853. DOI: 10.1021/acs.inorgchem.0c01353
Redox Flow Batteries
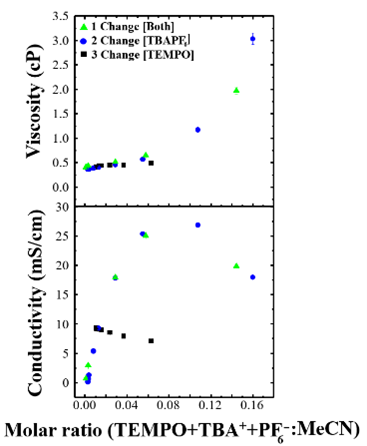
Redox flow batteries are an energy storage technology that stores generated energy in the form of electroactive species contained in liquid electrolytes. They are a versatile energy storage option due to their scalability, decoupled energy and power density, and design tunability. We are part of a multi-institutional collaborative grant for Data-enabled Discovery and Design to Transform Liquid-based Energy Storage (D3TALES). This research is focused on rapid organic electroactive candidate identification via database development, machine learning, and analytical automation, all supported by electrochemical characterization. We perform both electrochemical and spectro-electrochemical measurements on organic redox probes used in redox flow batteries to optimize experimental methodologies, determine quantitative information on these systems, and ultimately inform energy storage technology development.

| 
|
Stumme, N.; Perera, A. S.; Horvath, A.; Ruhunage, S.; Duffy, D. H.; Koltonowski, E. M.; Tupper, J.; Dzierba, C.; McEndaffer, A. D.; Teague, C. M.; Risko, C.; Shaw, S. K. Probing Redox Properties of Extreme Concentrations Relevant for Nonaqueous Redox-Flow Batteries. ACS Appl. Energy Mater. 2023, 6(5), 2819– 2831. DOI: 10.1021/acsaem.2c03712
Fluid Flow at Structured Surfaces
This project aims to develop the understanding of complex chemical and physical interactions between fluid molecules surfaces. For many years, empirical evidence has shown that a fluid will flow more slowly (or not at all) as it approaches a solid wall, such as the inside of a garden hose. This research project aims to discover exactly what the relationship is between fluid flow and wall roughness. To do this we will press the limits of nanofabrication techniques to create ultra-smooth surfaces and slowly increase roughness on a sub-nanometer scale while monitoring the fluid behavior. We predict (as do some others) that a critical threshold of roughness exists below which a fluid will flow as freely near the wall as it does far away from the wall. However, we are also cautious to consider the strength of possible intermolecular interactions between the fluid and the solid wall. The outcomes of this work will impact fundamental surface science as well as the foundations of fluid mechanics and the hydro-dynamic theory. The insights to be gained in surface-fluid and fluid-fluid interactions, the role of van der Waals forces, hydrogen bonding, and micro-viscosity, will be immediately useful to all areas involving fluid flow, particularly water filtration, microfluidics, biomedical devices, and countless surface preparation techniques.
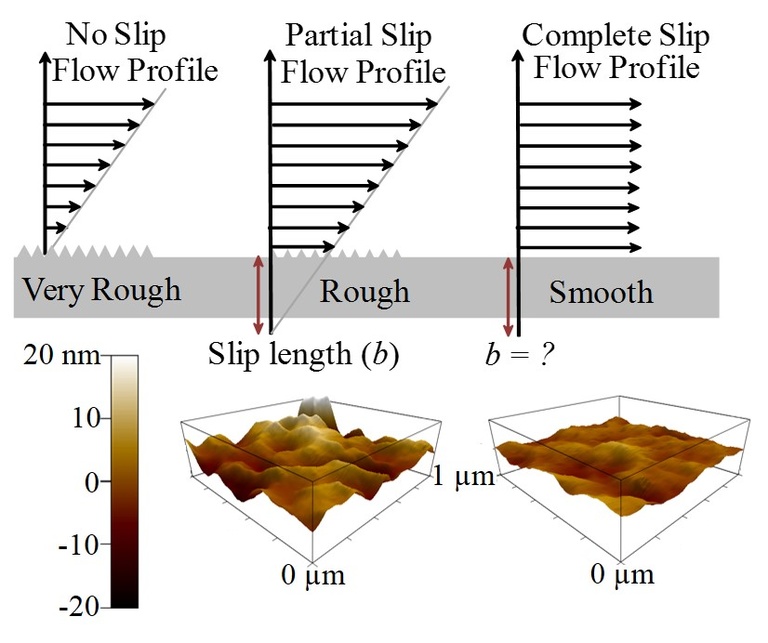
Nania, Samantha L.; Shaw, Scott K. Analysis of fluid film behaviour using dynamic wetting at a smooth and roughened surface. Analytical Methods Advance Article. DOI: 10.1039/C5AY00574D
Ionic Liquid Interfaces
ION ORIENTATION
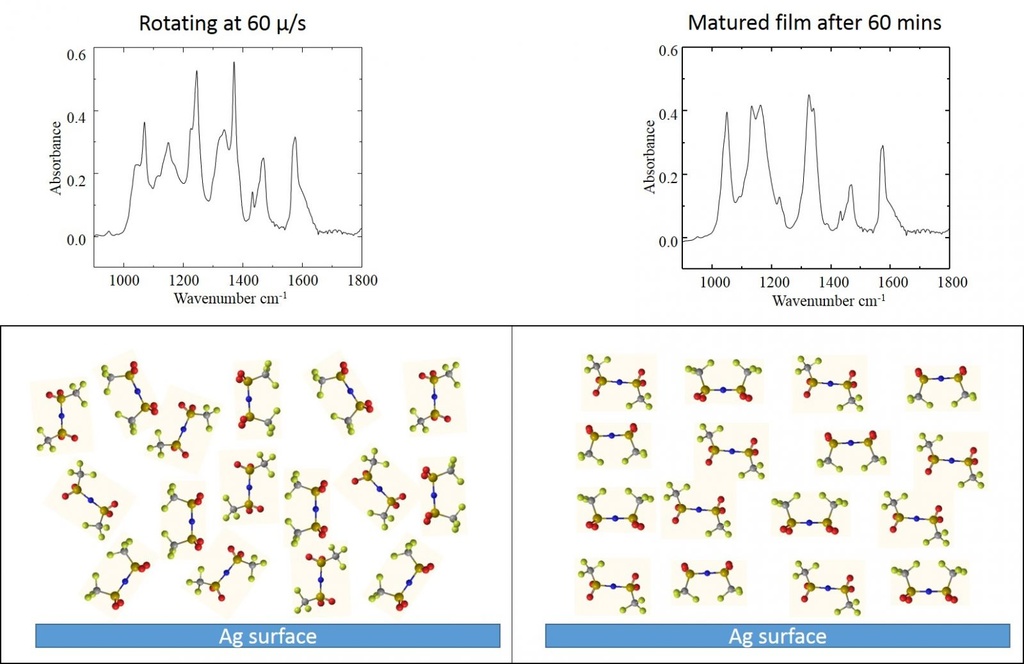
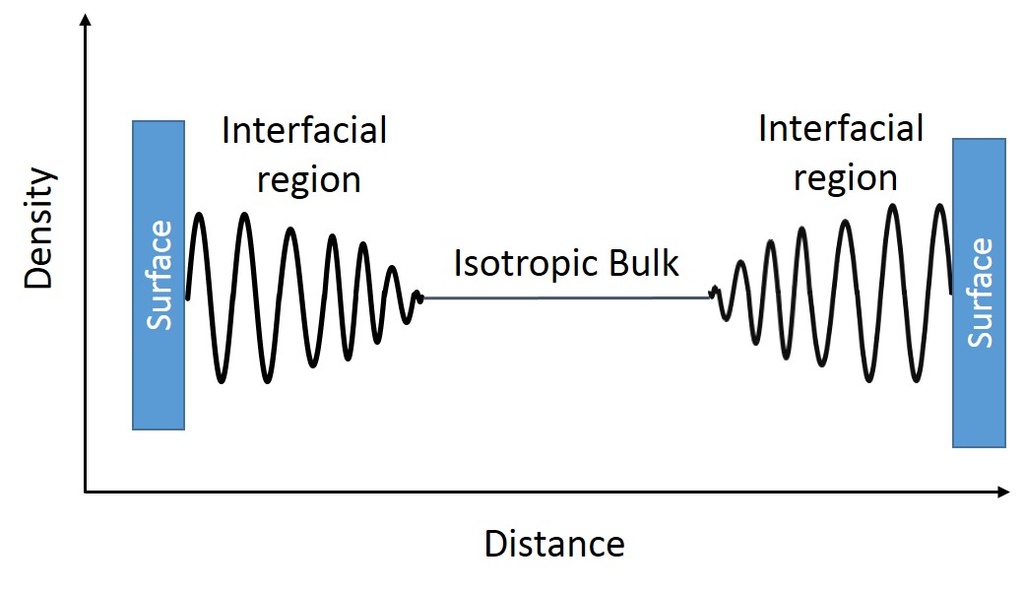
Ionic liquids exhibit low vapor pressure, high thermal stability, and electrical conductivity. They are of interest for applications in electrochemistry, catalysis, lubrication, photovoltaics, etc. Their macroscopic properties in the above-mentioned application environments are governed by their orientation and behavior at the solid surface. This study aims at studying IL films on a silver substrate using spectroscopic techniques.
Previous studies on IL interface show ordering at the solid-liquid interface which extends up to a few tens of nanometers and is characterized by oscillatory density profiles as shown in the above figure. Beyond this region bulk isotropic characteristics of the fluid are observed. Our results indicate high molecular ordering throughout the film which extends up to a few microns. Our result of the rearrangement of bis(trifluoromethylsulfonyl)imide anion as evidenced by the change in the IRRAS spectra.
|
|
THERMAL BEHAVIOR OF IONIC LIQUIDS AND THEIR MIXTURES
IL properties are strongly influenced by the addition of a cosolvent yet the effects of dilution on the phase change behavior of IL-mixtures and IL-solid interface is poorly understood. This project aims to investigate the complex phase change behavior of ILs and IL mixtures by using calorimetry and spectroscopy to study types of phase transitions, the temperatures they occur, and the resulting physical structure. This is important as these affect IL application performances as many IL applications, such as electrochemistry, lubrication, and space technologies, are used over wide temperature ranges.
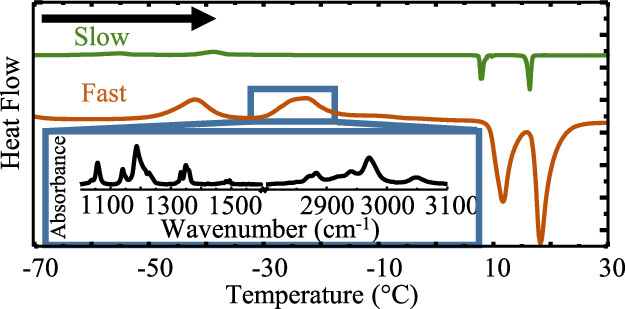
Curry, J.N.; Shaw, S.K. Thermotropic Phase Transitions in Butyltrimethylammonium Bis(trifluoromethylsulfonyl)imide Ionic Liquids are Dependent on Heat Flux. J Phys Chem B. 2019, 123 (22), 4757-4765. DOI: 10.1021/acs.jpcb.9b01650
CAPACITANCE STUDIES ON IONIC LIQUIDS
This project aims to study the relationship between an ionic liquid's capacitance and its' other properties such as viscosity, density, and ion orientation at an electrode surface. These studies are accomplished using a variety of techniques such as vibrational sum frequency generation spectroscopy (vSFG) as well as various rheological and electrochemical impedance analyses.
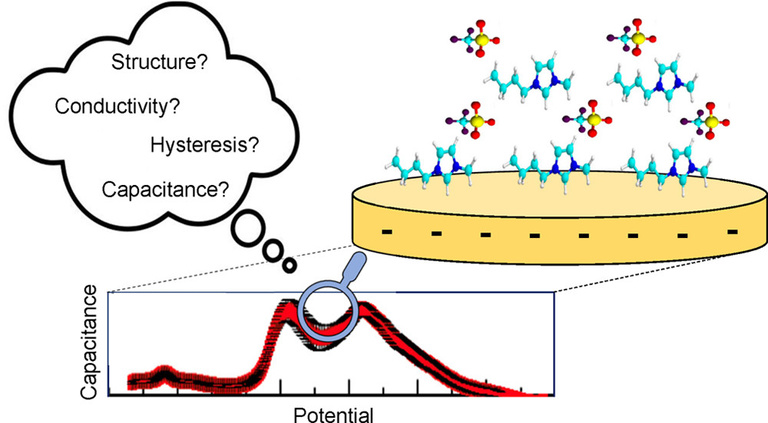
Pitawela, Niroodha R.; Shaw, S.K. Capacitive Hysteresis Effects in Ionic Liquids: 1-Ethyl-3-methylimidazolium Trifluoromethanesulfonate on Polycrystalline Gold Electrode. J. Electrochem. Soc. 2021, 168, 046510. DOI: 10.1149/1945-7111/abf4ac
Pitawela, Niroodha R.; Shaw, Scott K. Imidazolium Triflate Ionic Liquids’ Capacitance–Potential Relationships and Transport Properties Affected by Cation Chain Lengths. ACS Measurement Science Au. 2021, 1 (3), 117-130. DOI: 10.1021/acsmeasuresciau.1c00015
Environmental Film Studies
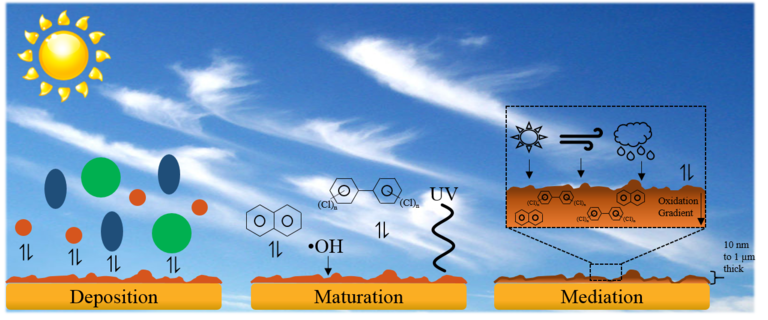
Environmental films are found on virtually every surface in a city’s landscape and act as sponges for atmospheric molecules and particulates, including persistent organic pollutants (POP). The films are generally composed of soot, water, and ‘grime’ that is emitted from various natural and man-made sources.
Our interest in these films is two-fold: to investigate the molecular level interactions that makes them efficient as chemical sponges, and to learn how these films interact with their environments to accumulate, mature, and release problematic chemicals into the environment. Our expertise in surface analysis will allow for groundbreaking research in this area relevant to atmospheric science, environmental chemistry, and corrosion science.

|
|
Grant, Jacob S.; Shaw, Scott K. A model system to mimic environmentally active surface film roughness and hydrophobicity. Chemosphere 2017, 185, 772-779. DOI: 10.1016/j.chemosphere.2017.07.068
Carbon Dioxide Recycling
The goal of this work is to capture atmospheric carbon dioxide and develop an environmentally responsible technology to recycle it back into valuable materials or fuels. The electrochemical reduction of CO2 in ionic liquids holds several distinct advantages, including a high solubility of CO2, an extended range of electrochemical stability, and nearly infinite tunability of the ionic liquid as a solvent / charge carrier. This work will involve a multi-pronged approach including advanced spectroscopic methods, electrochemical analysis, theoretical simulations, and synthesis of new electrodes and ionic liquid materials.
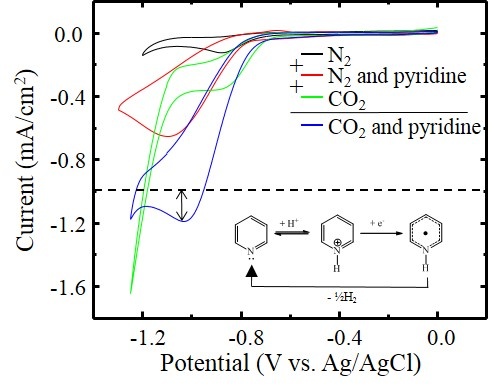
Lucio, Anthony J.; Shaw, Scott K. Pyridine and Pyridinium Electrochemistry on Polycrystalline Gold Electrodes and Implications for CO2 Reduction. Journal of Physical Chemistry C. 2015, 119 (22), 12523-12530. DOI: 10.1021/acs.jpcc.5b03355